Breast cancer (BC) is the most common cancer of women worldwide, accounting for 16% of all cancer cases in women (WHO).
 The most frequent malignant neoplasms in the republic in 2011 were breast cancer (11.6%), 3,515 people. Breast cancer is the most common cancer in the female population (21.4%), followed by skin tumors (11.6%), cervix (8.8%), ovarian (5.7%).
The most frequent malignant neoplasms in the republic in 2011 were breast cancer (11.6%), 3,515 people. Breast cancer is the most common cancer in the female population (21.4%), followed by skin tumors (11.6%), cervix (8.8%), ovarian (5.7%).
Breast cancer is a multifactorial disease, the development of which is associated with both environmental factors and individual genomic characteristics.
BRCA1, BRCA2 genes
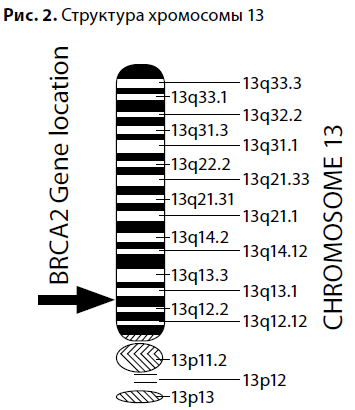
The predisposition to breast cancer became an area of active research, marked in the 1990s by the discovery of the BRCA1 gene on the long arm of chromosome 17 (locus 17q21), and then the BRCA2 gene on the long arm of chromosome 13 (13q12-13). Mutations of the BRCA genes were found to be inherited by autosomal dominant type with incomplete penetrance. 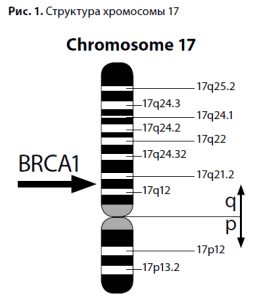 These discoveries had a "domino effect" for fundamental biology and genetics: low-penetrant genes, such as PTEN, P53, ATM, CHEK2, FANC, NBS1, responsible for the development of hereditary cancer syndromes in 5-15% of cases were subsequently discovered.
These discoveries had a "domino effect" for fundamental biology and genetics: low-penetrant genes, such as PTEN, P53, ATM, CHEK2, FANC, NBS1, responsible for the development of hereditary cancer syndromes in 5-15% of cases were subsequently discovered.
The Human Gene Mutation Database is continually updated with new mutation variants, and currently contains data on more than 1,400 mutations in the BRCA1 gene and about 1,100 mutations in the BRCA2 gene.
Numerous studies show that mutations in BRCA1 not only lead to a high lifetime risk of developing ovarian cancer, but also impose features on its clinical course. Carriers of the damaged BRCA1 gene often have an early onset of ovarian cancer, with primary multiple tumors involving both the ovaries and the breast. The family history of such patients is characterized by the presence of breast and ovarian cancer in blood relatives. Patients with BRCA1-associated ovarian cancer in 80% of cases have serous adenocarcinoma which is diagnosed at the average age of 48 years. BRCA1-associated ovarian cancer may have a better prognosis than sporadic ovarian cancer.
Mutations in the BRCA1 gene have the following characteristics:
1. Increase the lifetime risk of breast cancer by up to 65%;
2. Increase 40 to 60% lifetime risk of developing a second breast cancer;
3. Increase up to 39% lifetime risk of developing ovarian cancer;
4. Increase the risk of other malignant epithelial tumors (e.g., prostate cancer, stomach cancer).
 BRCA2 gene disorders have been identified in families where no association of breast and ovarian cancer syndrome with mutations in the BRCA1 gene has been found. BRCA2 is also a tumor suppressor gene by function. In contrast to BRCA1, BRCA2 mutations can be not only generative but also somatic and can be detected in the late stages of sporadic ovarian cancer. BRCA1 and BRCA2 gene products are involved in various cellular processes, mainly related to activation of transcription and DNA repair. For example, it has been shown that cells with an inactivated BRCA2 gene are characterized by increased sensitivity to mutagens and more intense accumulation of chromosomal damage
BRCA2 gene disorders have been identified in families where no association of breast and ovarian cancer syndrome with mutations in the BRCA1 gene has been found. BRCA2 is also a tumor suppressor gene by function. In contrast to BRCA1, BRCA2 mutations can be not only generative but also somatic and can be detected in the late stages of sporadic ovarian cancer. BRCA1 and BRCA2 gene products are involved in various cellular processes, mainly related to activation of transcription and DNA repair. For example, it has been shown that cells with an inactivated BRCA2 gene are characterized by increased sensitivity to mutagens and more intense accumulation of chromosomal damage
Mutations in the BRCA2 gene have the following effects:
1. Increase up to 45% lifetime risk of breast cancer in women and up to 6% in men;
2. Increase the lifetime risk of ovarian cancer by up to 11%;
3. Increase the risk of other malignancies such as melanoma, laryngeal cancer, pancreatic cancer, and stomach cancer.
With regard to malignant neoplasms of other localizations, it has been shown that gastric, uterine, and lung cancers occur more frequently in families with BRCA1 mutations; a high incidence of gastric and colorectal cancers is observed among relatives of probands carrying mutations in the BRCA2 gene. An earlier age of neoplasia in BRCA1 carriers has been noted [1]. The lifetime likelihood of developing breast cancer in women who are carriers of BRCA1 or BRCA2 mutations is extremely high.
A. Antoniou et al. (2003) analyzed 22 studies including 8,136 women with a family history of BC and/or ovarian cancer, 500 of whom were carriers of the pathological BRCA genotype. The authors concluded that the cumulative risk of developing BC by the age of 70 years was 65% for women with the BRCA1 mutation and 45% for those with the BRCA2 mutation (Figure 3, 4). However, if a family member under the age of 35 is diagnosed with breast cancer, the cumulative risk reaches 87% at the age of 70 for BRCA1 mutation carriers and 55% for BRCA2 mutation carriers.
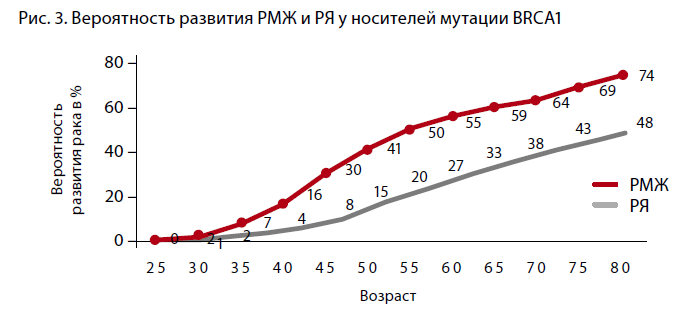
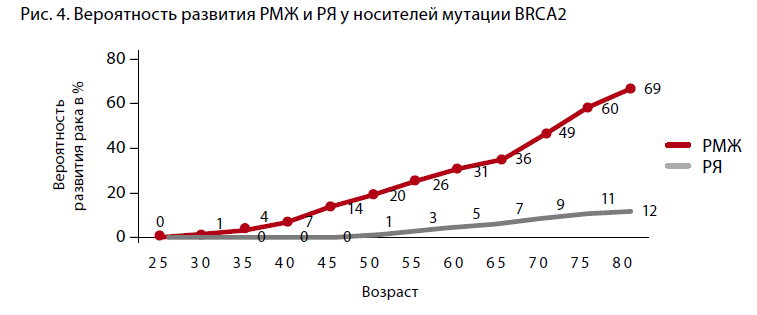 The absence of mutations in the most studied BRCA 1, 2 genes in families with a high incidence of ovarian and/or breast cancer does not exclude the presence of hereditary ovarian cancer due to unknown generative mutations, which are the subject of research to date [2]. The prevalence of mutations varies in different geographic regions. For example, the BRCA1 gene mutation 5382insC is the most common in Eastern Europe, with a frequency of 58.6% in Latvia, 63.6% in Estonia, and 51% in Poland. Mutations with a high frequency occurring in individual populations are known to be fundamental. For Slavic populations, this is the 5382insC mutation, which occurs in 70-80% of Russian patients. Lubchenko et al. (2003) reported an association of hereditary BC with the 5382insC mutation in 78.5% of cases [3].
The absence of mutations in the most studied BRCA 1, 2 genes in families with a high incidence of ovarian and/or breast cancer does not exclude the presence of hereditary ovarian cancer due to unknown generative mutations, which are the subject of research to date [2]. The prevalence of mutations varies in different geographic regions. For example, the BRCA1 gene mutation 5382insC is the most common in Eastern Europe, with a frequency of 58.6% in Latvia, 63.6% in Estonia, and 51% in Poland. Mutations with a high frequency occurring in individual populations are known to be fundamental. For Slavic populations, this is the 5382insC mutation, which occurs in 70-80% of Russian patients. Lubchenko et al. (2003) reported an association of hereditary BC with the 5382insC mutation in 78.5% of cases [3].
In contrast to other populations with a relatively high frequency of detecting mutations in the BRCA1/2 genes, the study by Akilzhanova A.R. (Astana, 2006) found no underlying mutations. The need for further research on genetic screening of BRCA1/2 mutations in the population of Kazakhstan, to determine the most frequent polymorphisms is obvious [4].
CHEK2 gene
The CHEK2 gene is also a suppressor of tumor growth, located on chromosome 22 and is involved in maintaining genome integrity.
It is involved in maintaining genome integrity with kinase activity and phosphorylates many proteins, in particular p53 and BRCA1. The CHEK2 gene is involved in protective reactions such as cell cycle arrest, DNA repair and apoptosis. CHEK2 mutations first identified have been associated with the LiFraumeni syndrome (characterized by an extremely invasive familial cancer phenotype usually associated with inherited mutations in the p53 gene). In European countries, it occurs with a frequency of 0.2% to 1.4% and determines a 2 to 10-fold increase in the risk of developing breast cancer. This mutation is also associated with an increased risk of tumors of other localizations (ovarian, prostate, colon, stomach), and may occur in "cancer families" without BRCA1 and BRCA2 genetic defects. The frequency of the SNEC2 1 lOOdeIC mutation varies significantly among ethnic groups and different populations. The genetic defect SNEK2 ivs2+lG>A is considered to be specific to the Slavic population, is the most common (0.48%) and has been studied in the Polish population, leads to the formation of a truncated protein, is associated with BC, prostate cancer, and thyroid cancer.
Genetic counseling
Advances in oncogenetics make it possible to effectively address the issues of prevention and early diagnosis of breast cancer, as well as to form
The genetic counseling of patients at risk of developing breast cancer is optimal and meaningful for the patients concerned.
Currently, DNA diagnosis of BRCA1/2, TP53, CHEK2 genes is optimal and significant when counseling patients from risk groups of breast cancer development. The U.S. agency U. S. Preventive Service Task Force (USPSTF) has not recommended routine routine screening of women for BRCA1 and BRCA2 mutations. An indication for this study was developed.
Indications for genetic testing:
- If there have been at least three cases of breast or ovarian cancer in the subject's family, regardless of age at the time of the cancer diagnosis;
- if there was at least one case of bilateral (bilateral) breast cancer in the subject's family among close relatives;
- If there have been cases of breast cancer in the subject's family before the age of 42;
- Having two first-degree relatives with breast cancer, one of whom was diagnosed before age 50;
- the presence of both breast and ovarian cancer in first- and second-degree relatives;
- Having two or more first- or second-line relatives with ovarian cancer, regardless of age at the time of diagnosis;
- Having a first-degree relative with both breast and ovarian cancer;
- Having a male relative with breast cancer;
- Jewish descent (Ashkenazi) and having one first-degree relative or two or more second-degree relatives on the same side with breast or ovarian cancer;
It is extremely important to know that detection of a mutation is not a fatal sentence. On the contrary, the identification of the mutation allows the physician and the patient to take effective measures to prevent the development of the disease.
Recommendations for prevention of BC/RBC in carriers of BRCA mutations, developed by the National Cancer Institute (USA)
- Breast self-examination - from 18-20 years of age (monthly).
- Clinical examination - of breasts and ovaries - from 25-35 years old (once or twice a year).
- Mammography - from the age of 35 (annually).
- Ultrasound examination - from 25-35 years old (once or twice a year).
- NMR tomography - from 25-35 years old (annually).
- CA 125 monitoring - from 25-35 years old as indicated.
- Prophylactic mastectomy (with reconstruction) - as indicated.
- Prophylactic oophorectomy (in postmenopause) - as indicated .
Analysis to detect the most common mutations of BRCA1, BRCA2, CHEK2 is carried out in the genetic laboratory "Trigen", the following mutations are examined:
1) BRCA1 mutation-1 185delAG. Risk of breast cancer (BC) and ovarian cancer (OC);
2) mutation- 2 BRCA1 5382insC. Risk of breast cancer and ovarian cancer (OC); 2) mutation - 2 BRCA1 5382insC;
3) mutation - 3 BRCA1 Cys61Gly. Risk of OC;
4) mutation - 4 BRCA1 4153delA. Risk of BC and OC;
5) mutation - 1 BRCA2 6174delT. Risk of BC and OC;
6) mutation - 1 kinase control 1100 delC cell cycle point CHEK2. Risk of BC;
7) mutation - 2 kinase of control IVS2+1G>A cell cycle point of CHEK2. Risk of BC.
Biomaterial for the test is venous blood with EDTA stabilizer. No prior preparation of the patient is required.
Literature
1. Vergeichik G.I. Hereditary ovarian cancer from the position of oncogenetics.
2. Portnoy S.M. et al. Features of BRCA-associated breast cancer and methods of prevention of hereditary forms of breast and ovarian cancer. Russian Cancer Research Center named after N.N. Blokhin RAMS, Moscow, November 23-25, 2010. С. 93-99.
A. Budik et al. Genetically determined breast cancer: specific features and surgical prevention. "Bulletin of N.N. Blokhin Russian Cancer Research Center of the Russian Academy of Medical Sciences, vol. 23, no. 2, 2012.
Screening of BRCA1/2 gene mutations in women diagnosed with breast cancer in Kazakhstan. XIV Russian congress of oncology, materials, November 23-25, 2010, Moscow.
5. Krylova N.Y. The role of mutations in the SNEK2 gene in the formation of hereditary predisposition to the development of ovarian cancer.
Author's abstract of the dissertation for the degree of candidate of medical sciences. St. Petersburg, 2009.
Kazakhstan Medical Journal #4 (34) July-August, 2013
Kochetkova E.O,
Degemerzanova N.K.
TreeGene Molecular Genetics Laboratory